作者简介:蔡志文(1994—),深圳大学硕士研究生.研究方向:SPR传感系统搭建与应用研究.E-mail:994327664@qq.com
中文责编:方 圆; 英文责编:溯 心
1)深圳大学物理与光电工程学院,光电器件与系统教育部/广东省重点实验室,广东深圳 518060; 2)湖南省计量检测研究院,湖南长沙 410014
1)College of Physics and Optoelectronic Engineering, Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, Shenzhen University, Shenzhen 518060, Guangdong Province, P.R.China2)Hunan Institute of Metrology and Test, Changsha 410014, Hunan Province, P.R.China
optics; surface plasmon resonance sensing; kinetic analysis; protein A; protein G; antibody
DOI: 10.3724/SP.J.1249.2021.01098
将自研的基于波长扫描的表面等离子体共振传感系统与动力学分析方法相结合,研究葡萄球菌蛋白A、链球菌蛋白G与免疫球蛋白G(immunoglobulin G, IgG)的相互作用关系.实验测得,蛋白A和蛋白G与IgG的结合速率常数分别为1.3×105 L·mol-1·s-1和5.0×104 L·mol-1·s-1,说明蛋白A与IgG的结合效率更高.同时测得蛋白A和蛋白G与IgG的解离速率常数分别为2.1×10-2 s-1和5.0×10-3 s-1,说明蛋白G与IgG形成的复合物更稳定.此外,由解离速率常数与结合速率常数的比值得出蛋白G对IgG的亲和力较大.实验结果可为优化抗体固定方法提供必要参考和理论支撑.
We study the interactions between staphylococal protein A or streptococcus protein G and immunoglobulin IgG, respectively, based on the self-developed wavelength-based surface plasmon resonance(SPR)sensing system combined with the kinetic analysis. The experimental results show that the association rate constants ka of protein A and protein G with IgG are 1.3×105 L·mol-1·s-1 and 5.0×104 L·mol-1·s-1, indicating that the protein A binds to IgG more efficiently. At the same time, the dissociation rate constants kd of protein A and protein G from IgG are detected to be 2.1×10-2 s-1 and 5.0×10-3 s-1, indicating that the complex formed by protein G and IgG is more stable. In addition, the ratio of kd to ka indicates that protein G has higher affinity for IgG. This study may provide necessary reference and important theoretical support for the optimization of antibody immobilization methods.
免疫传感器是一种利用抗体和抗原之间亲和作用,实现目标分析物特异性检测的装置.在免疫传感器的制备中,由于抗体作为识别元件提供了抗体和抗原相互作用的特异性位点,抗体的固定效果对抗原检测至关重要.共价法是最常见的固定方法,但其通常比较耗时,且会导致抗体的随机固定[1].彼此接近的抗体会引起空间位阻效应,进而影响抗体对抗原的结合能力.利用抗体结合蛋白对抗体的特异性吸附可实现抗体的定点固定.葡萄球菌蛋白A(简称蛋白A)和链球菌蛋白G(简称蛋白G)可以特异性地与抗体的可结晶(fragment, Fc)段结合[2].然而,目前鲜有评估和比较两者与抗体结合效率的文章.
研究分子间相互作用的一般方法包括放射免疫分析法和酶联免疫分析法[3]等,但这些方法通常需要标记,具有数据分析复杂、不能实时监测反应过程的缺点.动力学分析法可实时监测分子间的结合和解离过程,表面等离子共振(surface plasmon resonance, SPR)传感技术无需对分析物进行标记,可实现高通量的生物分子间相互作用研究.自1990年Biacore公司开发出首台商品化的SPR检测仪器,SPR传感已成为研究分子间相互作用的重要工具[4-5].SPR传感检测分为强度型、相位型、角度型及波长型方法.强度型SPR传感器的检测装置结构简单,但是动态范围一般只能达到 ~10-5 RIU[6],灵敏度低; 相位型SPR传感器具有较高灵敏度[7],但其动态范围较小(~10-4 RIU); 角度型SPR传感器具有较高的灵敏度(~10-7 RIU)和动态范围[8],但是检测设备相对复杂和昂贵[9-11]; 波长型SPR传感器与强度型相比,其灵敏度更高,能够达到~10-6 RIU[12-13],且动态范围更大[14].波长型SPR检测设备的成本与角度型相比较低,因此,更适用于研究分子间的相互作用.本研究利用自行研制的波长型SPR生物传感设备,结合动力学分析法研究蛋白A、蛋白G与免疫球蛋白(immunoglobulin G, IgG)的相互作用.实验测得IgG与蛋白A和蛋白G特异性结合的动力学常数(结合速率常数和解离速率常数),比较了两者与IgG的结合能力.研究结果可为抗体固定方法优化提供必要科学参考.
1.1 主要试剂重组蛋白A购于上海市普欣生物科技有限公司,重组蛋白G购于生工生物工程(上海)股份有限公司,IgG(人种属)购自北京索莱宝科技有限公司, 1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride,EDC)、N-羟基琥珀酰亚胺(N-hydroxysuccinimide,NHS)和2-吗啉乙磺酸-水合物(2-morpholine ethanesulfonic acid hydrate, MES)均购自上海麦克林生化有限公司,巯基十一酸(mercaptoundecanoic acid, 11-MUA)和牛血清白蛋白(bovine serum albumin, BSA)购自上海阿拉丁生化科技有限公司,磷酸盐缓冲液(phosphate buffer saline, PBS)购于上海双螺旋生物科技有限公司.
SPR成像(SPR imaging, SPRi)传感实验装置如图1所示.卤素灯光源产生宽谱光经过透镜组L1和L2耦合进入液芯光纤,光纤出射光经过透镜L3准直后进入声光滤波器(acoustic optical tunable filter, AOTF).AOTF会对宽谱光进行调制,滤波输出窄带光.经AOTF出射的窄带光经柱面镜CL1和CL2准直扩束为椭圆型光斑倾斜入射到棱镜,以匹配棱镜的空间色散,提高光能利用率.光线经过棱镜耦合后入射到传感金膜,入射光在金膜表面激发SPR现象.最后反射光经过透镜组L4和L5成像至电荷耦合元件(charge-coupled device, CCD)相机的传感面.AOTF和CCD由LabVIEW软件控制,在AOTF切换1次波长后,CCD进行多次曝光得到多幅传感面强度图,平均后得到该波长下的传感面强度图.AOTF扫描1个波长周期后,获得一系列不同波长下对应的传感面强度图像.由于芯片不同点位的金膜厚度并不完全一致,在实际检测时,首先选取大小为30×20 pixel的感兴趣区域,以减小不同金膜厚度所引起的误差.对该区域像素点在每个扫描波长下的强度值进行平均,从而得到该区域的SPR光谱曲线,通过多项式拟合得到该区域的共振波长.金膜表面发生分子结合时会引起金膜与溶液界面的折射率变化,导致共振波长发生移动,由此监测分子间的相互作用过程.
为防止共振波长超出扫描范围,将系统的波长扫描范围设定为600~700 nm.系统中AOTF作为分光器件,最小光谱分辨率为0.1 nm,即扫描步长最小可达0.1 nm.在扫描步长分别为0.1、1.0 和10.0 nm情况下,系统对同种样品的均方根噪声分别为0.007、0.007和0.013 nm.因此,可认为扫描步长为0.1 nm和1.0 nm时的噪声水平基本一致,为提高系统的时间分辨率,实验选取扫描步长为1.0 nm.
对待测样品的折射率进行估计,通过理论模拟计算出最佳的入射波长以及入射角.如本次实验样品的背景液为PBS(折射率为1.334 7 RIU)时,对应的最佳入射波长和入射角分别为662 nm和71.1°.检测过程中首先固定最佳入射波长,在预估的最佳入射角附近调整入射角度,并对待测位点的强度值进行监测,当待测位点的强度值最低时,认为此时的入射角为最佳入射角,然后固定该角度,对入射波长进行扫描.
SPRi系统中使用的棱镜由折射率为1.515的BK7玻璃制成.传感芯片通过磁控溅射法制备,首先,在18 mm×18 mm的BK7玻璃基板上溅射2 nm厚的Cr黏合层; 然后,溅射48 nm厚的金传感层,实验中使用折射率匹配油将传感芯片与棱镜耦合.
实验中使用4通道流通池.流通池的模具由Solidworks制图设计,使用计算机数控(computer numerical control, CNC)方法加工而成.将配好的聚二甲基硅氧烷(polydimethylsiloxane, PDMS)胶倒入模具中,在150 ℃恒温箱中固化15 min.流通池尺寸为18 mm×18 mm,与传感器芯片的尺寸匹配.每个通道的体积约为3 μL(高度0.2 mm; 宽度1 mm; 长度14 mm).
首先,将金膜放置在等离子体清洗机内清洗10 min以增加金膜的亲水性,取出冷却后置于10 mL的11-MUA乙醇溶液中浸泡过夜制备自组装膜.利用无水乙醇和纯水清洗金膜表面,氮气吹干.将修饰好的金膜置于棱镜之上,在金膜表面固定多通道流通池.将PBS缓冲液流入流通池,待SPR信号稳定后,再通入含有0.2 mol/L EDC和0.1 mol/L NHS的MES溶液(0.1 mol/L,pH=5.5),活化金膜表面的羧基.接着通入含有100 μg/mL的蛋白A或蛋白G的醋酸钠溶液(0.1 mol/L,pH=4.5),抗体结合蛋白分子通过共价结合固定在芯片表面.最后通入质量浓度为1%的BSA溶液封闭未结合的NHS酯基.通入每种试剂前都要先通入PBS缓冲液对通道进行冲洗直至SPR共振波长稳定,以清洗掉金膜表面上的非特异性结合的蛋白分子.以上实验都在室温(20~25 ℃)下进行,液体进样速度为10 μL/min.
抗体结合蛋白固定后,在通道中通入PBS缓冲液直至信号稳定.将IgG用PBS溶液稀释成2个浓度组,第1组的质量浓度分别为1、2、4及8 μg/mL; 第2组的质量浓度分别为8、15、18及25 μg/mL.两组质量浓度梯度的IgG溶液分别通入固定了蛋白A和蛋白G的通道,IgG溶液在金膜表面与蛋白A和蛋白G发生结合反应.反应结束后通入PBS溶液进行解离.SPR传感器实时监测共振波长的变化,通过对共振波长变化曲线进行处理可得到相关动力学信息.
反应过程符合准一级动力学方程[15]
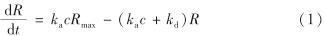
其中, dR/dt为 SPR信号对时间t的求导,即固定在金膜表面的抗体蛋白分子与抗体结合形成复合物的速率; Rmax为物质反应饱和时的 SPR信号; ka为结合速率常数; kd为解离速率常数; c为分析物的质量浓度(单位:mol/L).通过对dR/dt~R进行线性拟合,可求出斜率kac+kd; 再对(kac+kd)~c线性拟合,可求出斜率ka和截距kd. 若要求得更精确的kd,需从解离段数据中求取,解离段具有如下关系
(dR)/(dt)=-kdR(2)
对式(2)积分可得
ln(R0/Rt)=kd(t-t0)(3)
其中, R0与Rt分别是t0和t时刻的SPR信号.以ln(R0/Rt)~(t-t0)作图或线性拟合可求出斜率kd.
图2为蛋白 A和蛋白 G固定过程中共振波长的实时变化曲线.自组装金膜表面通入含有 0.2 mol/L EDC和 0.1 mol/L NHS的 MES溶液时,共振波长发生剧烈变化,这是由于 MES溶液与 PBS溶液较大的折射率差异所引起,金膜表面羧基活化后共振波长实际增加约 2 nm.抗体结合蛋白共价固定在金膜表面过程中共振波长逐渐增加, PBS冲洗后蛋白 A和蛋白 G结合区域共振波长分别提高 2.7 nm和 3.0 nm,说明抗体结合蛋白成功固定在金膜表面.BSA封闭后,共振波长进一步增加,表明 BSA在芯片上已固定.
图 3(a)和图 3(b)分别为蛋白 A和蛋白 G与 IgG反应的动力学曲线图.由式(2)和式(4)可以求出蛋白 A与 IgG的ka≈1.3×105 L·mol-1·s-1、kd≈2.1×10-2 s-1,蛋白G与 IgG的ka≈5.0×104 L·mol-1·s-1、 kd≈5.0×10-3 s-1.就结合速率相比,蛋白A与 IgG的结合速率更大,说明蛋白 A与 IgG结合更快,单位时间里结合形成更多的复合物,结合效率更高.就解离速率相比,蛋白 G与 IgG的解离速率更小,说明单位时间里解离程度小,形成的复合物相对稳定.重组蛋白 A有 5个 IgG的 Fc区域结合位点,重组蛋白 G则只有 2个或 3个[16-18],位点越多更容易结合,这可能是蛋白 A与 IgG能在单位时间里结合次数更多的原因.蛋白 A与 IgG的结合速率常数和解离速率常数与文献[19]中的量级一致,蛋白 G的结合速率常数与文献[20]中量级一致. kd/ka是体现亲和力大小的指标,比值越小则亲和力越强.蛋白 A与蛋白G相比kd/ka值较大,说明它对 IgG的亲和力较小.这可能是由于重组蛋白 G已经除去了与白蛋白的结合位点,减少了交叉反应与非特异性结合,因此,蛋白 G比蛋白 A具有更强的亲和力.
SPR传感技术是一种新型生化分析技术手段,被广泛应用于药物开发、环境检测和疾病诊断等领域.该技术能实时监测分子间的相互作用,如蛋白质-蛋白质、药物-蛋白质、核酸-蛋白质及受体-配体等分子间的相互作用.本研究利用自研的波长型SPR生物传感器研究蛋白A、蛋白G与免疫球蛋白IgG的相互作用,并对其进行动力学分析计算.结果表明,该传感器可用于研究生物分子相互作用及动力学参数测定,计算得到的动力学参数数量级与文献中相似.对比蛋白A和蛋白G与IgG的结合和解离速率参数可以得出,蛋白A与IgG的结合效率更高,而蛋白G与IgG形成的复合物更稳定,蛋白对IgG的亲和力更强.本研究结果为抗体固定方法的优化提供必要的参考和理论支撑.
深圳大学学报理工版
JOURNAL OF SHENZHEN UNIVERSITY SCIENCE AND ENGINEERING
(1984年创刊 双月刊)
主 管 深圳大学
主 办 深圳大学
编辑出版 深圳大学学报理工版编辑部
主 编 李清泉
国内发行 深圳市邮电局
国外发行 中国国际图书贸易集团有限公司(北京399信箱)
地 址 北京东黄城根北街16号
邮 编 100717
电 话 0755-26732266
0755-26538306
Email journal@szu.edu.cn
标准刊号 ISSN 1000-2618
CN 44-1401/N